Rsearchers from the East China Normal University (ECNU), the University of California (UC) San Diego, and the Second Military Medical University (SMMU) of China, identified a new molecular mechanism that contributes to the control of bowel inflammation.

Professor Li Xiao Tao (Left) works at the laboratory with his team members.
The REGγ-proteasome forms a regulatory circuit with IκBε and NFκB in experimental colitis, the research paper of their findings was published in Nature Communication on February 22, 2016. Professor Li Xiaotao from ECNU, Xiao Jianru from SMMU, and Professor Alexander Hoffmann form UC San Diego, are the corresponding authors of the paper.
Inflammatory bowel disease (IBD) is a worldwide health-care problem with significant morbidity. Including ulcerative colitis (UC) and Crohn’s disease (CD), IBD is characterized by chronic relapsing intestinal inflammation. IBD patients often have symptoms of diarrhea, abdominal pain, hematochezia and weight loss. It is now known that inflammatory diseases increase the risk of developing cancers. Colon cancer is the third most common cancer in males and the second in females worldwide. Statistics show there are at least five million IBD patients in the world and China has seen about 350,000 IBD cases in recent 10 years. Therefore, a better understanding of the predisposing factors and the underlying molecular mechanism of IBD is of great emergency.
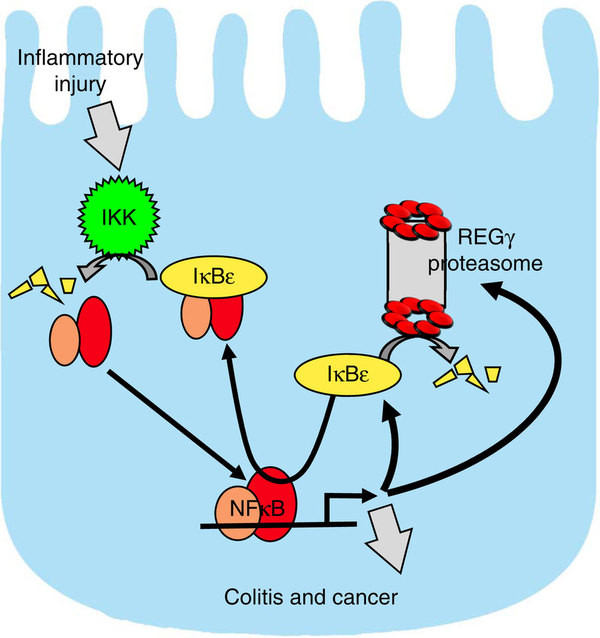
A schematic model depicts the reciprocal regulation between REGγ and NFκB.
In this paper, Professor Li and his partners demonstrate that mice deficient for REGγ, a proteasome activator, show significantly attenuated intestinal inflammation and colitis-associated cancer in dextran sodium sulfate model. Bone marrow transplantation experiments suggest that REGγ’s function in non-haematopoietic cells primarily contributes to the phenotype. Elevated expression of REGγ exacerbates local inflammation and promotes a reciprocal regulatory loop with NFκB involving ubiquitin-independent degradation of IκBε. Additional deletion of IκBε restored colitis phenotypes and inflammatory gene expression in REGγ-deficient mice. Their study identifies REGγ-mediated control of IκBε as a molecular mechanism that contributes to NFκB activation and promotes bowel inflammation and associated tumour formation in response to chronic injury.