On January 14, Nature Communication published“Bond cleavage, fragment modification and reassembly in enantioselective three-component reactions”, a paper of Prof. Hu Wenhao’s research team of Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, and Department of Chemistry, East China Normal University. This paper first reported “bond cleavage, fragment modification and reassembly”, an innovative mode of chemical bond formation to achieve challenging synthetic tasks.
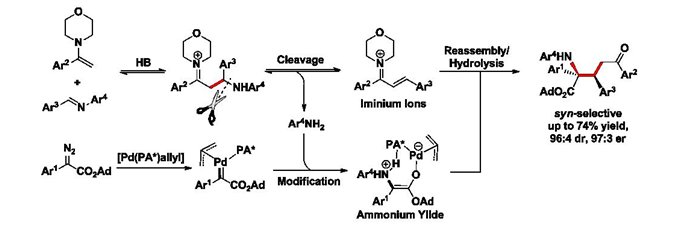
Actual process: bond cleavage, fragment modification and reassembly.
Chemical bond cleavage and reconstruction are common processes in traditional rearrangement reactions. Bond cleavage and reassembly of fragments in traditional two-component reactions have been reported, but in most cases, these reactions resulted in either loss of components or random combination of the fragments such as radical fragments without any selectivity. Therefore, innovative processes involving new modes of chemical bond formation are highly desirable to achieve challenging synthetic tasks that existing ones cannot easily accomplish.
Prof.Hu (Left) and his team members.
Prof. Hu and his team members have communicated a multi-component process that involves chemical bond cleavage, fragment modification and specific reassembly of the modified fragment to afford functional group-enriched molecules. This innovative process provides a unique way to build diversified molecules in an efficient and atom-economic fashion.
More information about the findings can be seen in the paper, “Bond cleavage, fragment modification and reassembly in enantioselective three-component reactions”.