Recently, the research team headed by Prof. Xu Lin from the School of Chemistry and Molecular Engineering made important progress in the precise self-assembly of complex architectures. The results have been published in the eminent journals Journal of the American Chemical Society and Angewandte Chemie International Edition. Moreover, one of them was selected as a “Very Important Paper”.
Design and construction of new functionalized supramolecular coordination complexes (SCCs) via coordination-driven self-assembly strategy is highly important in supramolecular chemistry and materials science. Xu’s group presented a family of well-defined metallacycles decorated with mesogenic forklike dendrons through the strategy of coordination-driven self-assembly (Fig. 1). Due to the existence of mesogenic forklike dendrons, the obtained metallacycles displayed the smectic A liquid crystal phase at room temperature while their precursors exhibited the rectangular columnar liquid crystal phase.
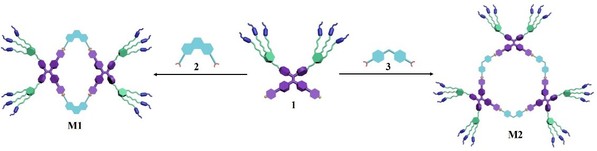
Fig. 1. The construction of discrete rhomboidal metallacycle M1 or hexagonal metallacycle M2 via coordination-driven self-assembly.
Interestingly, the prepared liquid-crystalline metallacycles could be further applied for holographic storage of colored images (Fig. 2). Notably, the rhomboidal metallacycle and hexagonal metallacycle gave rise to different holographic performances although they featured a similar liquid crystal phase behavior. Therefore, this research not only provides the first successful example of supramolecular liquid-crystalline metallacycles for holographic storage of colored images but also opens a new door for supramolecular liquid-crystalline metallacycles toward advanced optical applications. These results have been published in Journal of the American Chemical Society (J. Am. Chem. Soc., 2020, 142, 6285).
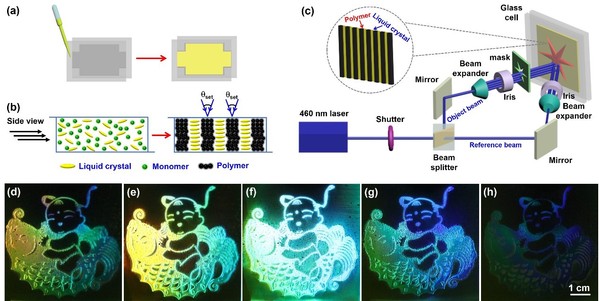
Fig. 2. Illustration of patterning holographic images.
Recently, Xu’s group expanded their research interests from two-dimensional supramolecular metallacycles to three-dimensional supramolecular metallacages. They used di(2‐pyridyl)ketone in subcomponent self‐assembly. When combined with a flexible triamine and zinc bis(trifluoromethanesulfonyl)imide, this ketone formed a new Zn4L4 tetrahedron 1 bearing twelve uncoordinated pyridyl units around its metal‐ion vertices (Fig. 3).
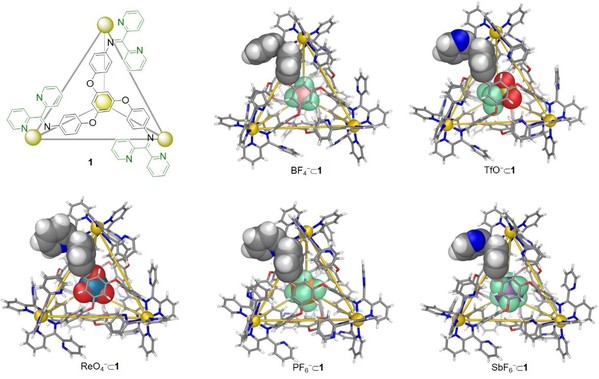
Fig. 3. The chemical structure of cage 1 and its crystal structures.
The acid stability of 1 was found to be greater than that of the analogous tetrahedron built from 2‐formylpyridine. Intriguingly, the peripheral presence of additional pyridine rings in 1 resulted in distinct guest binding behavior from that of the analogous tetrahedron built from 2‐formylpyridine, affecting guest scope as well as binding affinities. The different stabilities and guest affinities enabled the design of systems whereby different cargoes could be moved between cages using acid and base as chemical stimuli (Fig. 4). These results have been published in Angewandte Chemie International Edition (Angew. Chem. Int. Ed., 2020, 59, 7435).
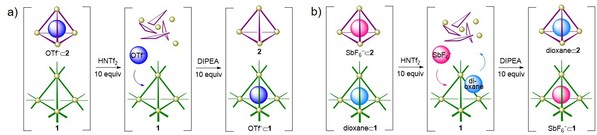
Fig. 4. Representation of transfer and exchange of cargoes between cages 1 and 2 upon sequential acid–base addition.
Subsequently, they reported a network of supramolecular transformations, based upon a subcomponent displacement strategy. The flow through this network is directed by the relative reactivities of different amines, aldehydes, and di(2-pyridyl)ketone. The network provides access to an unprecedented heteroleptic Cd6L6L′2 twisted trigonal prism. The principles underpinning this network thus allow for the design of diverse structural transformations, converting one helicate into another, a helicate into a tetrahedron, a tetrahedron into a different tetrahedron, and a tetrahedron into the new trigonal prismatic structure type (Fig. 5). The selective conversion from one host to another also enabled the uptake of a desired guest from a mixture of guests (Fig. 6). These results have been published in Journal of the American Chemical Society (J. Am. Chem. Soc., 2020, DOI: 10.1021/jacs.0c03798).
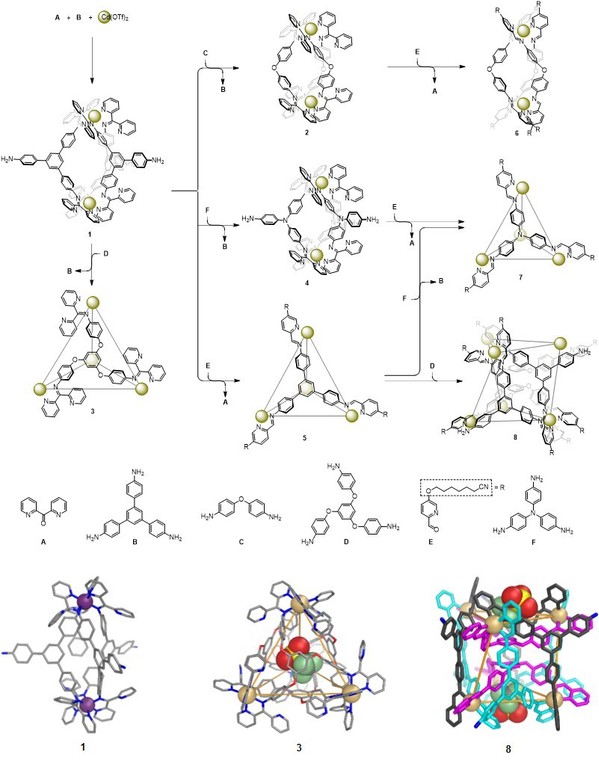
Fig. 5. Network of transformations between architectures 1–8
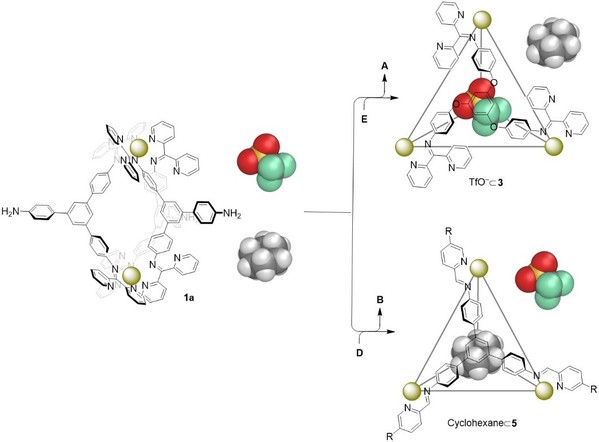
Fig. 6. Schematic representation of selective uptake of either triflate or cyclohexane upon structural transformations.
The above studies were cooperatively conducted with Prof. Peng Haiyan’s group from Huazhong University of Science and Technology and Prof. Jonathan Nitschke’s group from the University of Cambridge in the UK, and were also guided by Prof. Yang Haibo of ECNU. The research project was funded by the National Natural Science Foundation of China and Shanghai Pujiang Program.
Source: School of Chemistry and Molecular Engineering
Copy Editor: Philip Nash
Editor: Yu Wenxi