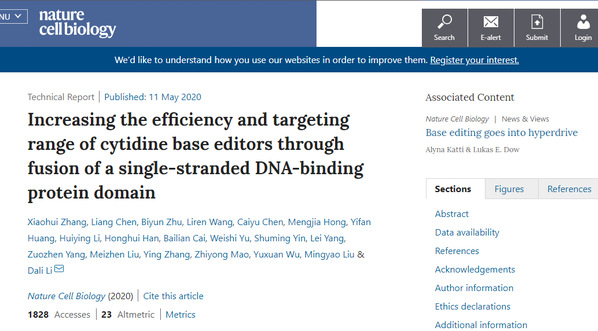
A series of new gene-editing tools have been developed by Prof. Li Dali’s research group from East China Normal University (ECNU), after their two years’ unremitting endeavor in this project. These new tools are calledhyCBEs(hyper activity cytosine base editors), which have proved themselves suitable for gene therapies for genetic diseases, especially those caused by single-base mutations. The study was published in the latest issue of the high-profile academic journal Nature Cell Biology.
“This new set of base editing tools not only has higher editing efficiency and a wider editing window but also maintains precise working performance,” said Li, chief scientist of the research group.
Accurate generation of animal disease models by hyCBE withhundred-fold increased activity
By fusing the single-stranded DNA binding domain of Rad51 protein between Cas9 and the deaminase of the cytosine base editor BE4max, Li and his group members created a new base editor with enormously improved editing activity and a broadened editing window. They named this new cytosine base editor hyper CBE (hyBE4max). Inspired by this distinctive strategy, they followed up to create hyA3A-BE4max and hyeA3A-BE4max. The former is endowed with a broader editing window and higher activity, and the latter can more efficiently edit Cs in a TC motif without bystander mutations.
“Taking hyeA3A-BE4max as an example, the editing activity of specific sites increases by up to 257-fold among the tested targets.” Li said, “Through embryo microinjection, the single base can be accurately altered under embryo level. The average base editing efficiency in F0 for hyeA3A-BE4max was 60-folder higher than that for eA3A-BE4max.”
In addition to animal models, another advantage is to leverage the hyeA3A-BE4max to treat genetic diseases, for example,β-thalassemia. Better potential therapeutic effects of this tool on β-thalassemia were observed when a single base was precisely mutated. It is also shown by a series of rigorous experiments that this new editor has a rather high accuracy without obvious DNA and RNA off-targets, demonstrating the great potential of the new editing tool for gene therapy.

Li Dali (right) and Zhang Xiaohui, a PhD student of the research group.
ECNU teamforges aheadin the field of genetherapy
In recent years, European and American countries have successively approved gene therapies for the treatment of genetic diseases such as adenosine deaminase (ADA) deficiency, severe combined immunodeficiency, spinal muscular atrophy,β-thalassemia, and Leber’s congenital melanosis. Due to technical limitations,currently marketeddrugs might still have flaws in long-termefficacy, or risks of carcinogenesis. Accordingly, scientists continue to develop new genetically modified technologies in order to overcome the deficiencies of existing technologies and achieveone treatment for lifelong cure.
The gene-editing technology represented by the CRISPR / Cas9 system invented in 2012 is one of the hottest molecular biology technologies in recent years. The research team of Liu Mingyao and Li Dali from the School of Life Sciences of ECNU took the lead in establishing the CRISPR/Cas9 system forgenerating disease models withhigh-speed in 2013, which shortened the time from 18 months to about 5 weeks. Subsequently, CRISPR/Cas9 technology was employed to modify single base mutation in animal models and cure hemophilia B, demonstrating its potential in the treatment of genetic diseases. However, the efficiency to accurately achieve base mutations via CRISPR/Cas9 mediated-homologous recombination is still low (lower than 1%), hindering the wider application of the strategy for gene therapy.
The CBE technology developed in 2016 by American scientists can directly convert cytosine into thymine within a certain range of the target fragment, avoiding the occurrence of DNA double-strand breaks. Early this year, Li’s research group published a paper regarding successfully establishing the single-base editing system in human hematopoietic stem cells via the CBE, proving that CBE has the potential to treat β-thalassemia. However, low editing efficiency and limited accuracy need tobe urgently solved.
“The base editing technology equipped with hyper activity reported in this paper might solve the above problems, which isexpected to become the preferred base editor-genetic diseases,” Li said.
ECNU graduate students Zhang Xiaohui, Chen Liang, and Zhu Biyun are co-first authors of this paper.Prof.Li Dali is the corresponding author of this study. Prof. Liu Mingyao provided guidance to this study.
To know more about the research:
https://www.nature.com/articles/s41556-020-0518-8
https://www.nature.com/articles/s41556-020-0521-0
Source: School of Life Sciences
Copy editor: Philip Nash
Editor: Yu Wenxi