# Hot Search #
On July 15, 2024, the achievements made by ECNU’s scientific research team, composed of Du Bing, Liu Mingyao, and Li Dali from the School of Life Sciences, were published online in Cell, a top international journal, marking the first global report on the use of allogeneic universal CAR-T to treat rheumatic immune diseases.

ECNU’s scientific research team composed of Du Bing, Liu Mingyao, and Li Dali from the School of Life Sciences

https://www.cell.com/cell/fulltext/S0092-8674(24)00701-3
Rheumatic immune diseases affect more than 8% of the population, featuring high morbidity rates, high disability rates, and high treatment costs. They are considered the third greatest threat to human health, following only cardiovascular diseases and cancer, and are referred to as the “immortal cancer”. Thus, improving the cure rate of rheumatic immune diseases, minimizing the prevalence and disability rate, and improving the living quality of patients have become the common medical challenge and focus worldwide, and should be addressed urgently.
Chimeric Antigen Receptor (CAR)-T cell therapy, as an innovative medical technology, has not only shown excellent efficacy in the treatment of B-cell hematological malignancies but also demonstrates promising results in the treatment of rheumatic immune diseases. However, CAR-T cells constructed from a patient’s autologous cells face significant challenges such as high production costs, long manufacturing times, complex processes and clinical safety, which have greatly limited their clinical accessibility. Therefore, allogeneic universal CAR-T cells, derived from healthy donors and capable of large-scale production, present the best solution to fundamentally reduce the high cost and poor clinical accessibility of existing CAR-T cell therapy products.
However, the development of allogeneic universal CAR-T cells has been one of the greatest technical challenges in the industry. Existing gene editing strategies can effectively destroy the TCR structure to prevent Graft-versus-Host Disease (GvHD) in patients, but they have not been able to effectively address the immune rejection issues faced by allogeneic CAR-T cells in patients. Traditional allogeneic universal CAR-T cells in clinical settings have consistently faced problems such as excessive immunosuppression, which brings significant infection risks to patients, and short invivo persistence with poor efficacy. These problems have greatly limited their clinical accessibility.
After eight years of painstaking research and hard work, ECNU’s research team of Du Bing, Liu Mingyao, and Li Dali from the School of Life Sciences creatively developed a brand-new strategy of allogeneic universal CAR-T cells—TyU19 using gene editing technology, which can effectively solve the above-mentioned problems. In clinical treatment, it has achieved long-term persistence and effective B cell killing in patients, and its clinical safety, effectiveness, and other indicators have reached or even surpassed traditional autologous CAR-T cells.
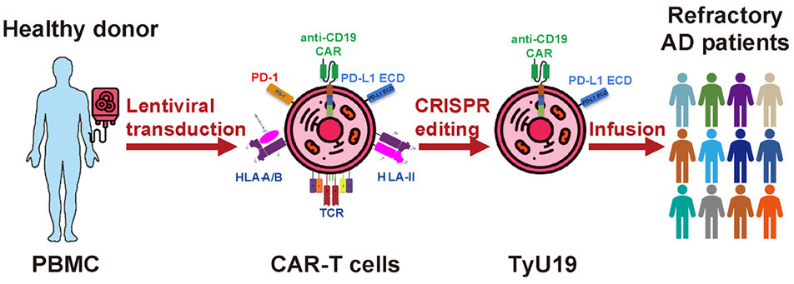
Preparation strategy of allogeneic universal CAR-T cells—TyU19
Based on previous research results, the medical team led by Prof. Xu Huji from Changzheng Hospital of Naval Medical University pioneered the application of TyU19 in the treatment of three patients with severe relapsed and refractory rheumatic immune diseases.
The results showed that universal CAR-T cells targeting CD19 could effectively expand and completely eliminate B lymphocytes in all patients, leading to the reconstitution of B cells after three months. In patients with immune-mediated necrotizing myopathy (IMNM), their muscle strength improved significantly. MRI and pathology showed a marked reduction in muscle inflammation. Laboratory tests indicated that muscle enzymes dropped from abnormally high levels tonormal, and autoantibodies were completely cleared. For patients with diffuse systemic sclerosis (SSc), their skin was softened, accompanied by improvement in inflammation and the formation of new accessory glands, as confirmed by skin biopsies. Moreover, CT and MRI revealed a reversal of fibrosis in vital organs such as the heart and lungs, with continued improvement observed during the six-month follow-up period.
Furthermore, clinical observations and laboratory tests confirmed the good safety profile of the universal CAR-T cells targeting CD19. The results of this pioneering clinical study show that allogeneic CD19 CAR-T cells can effectively treat patients with refractory and relapsed rheumatic immune diseases who have failed in existing treatments and are well tolerated, demonstrating that universal CAR-T cells—TyU19 have huge therapeutic potential and market value.
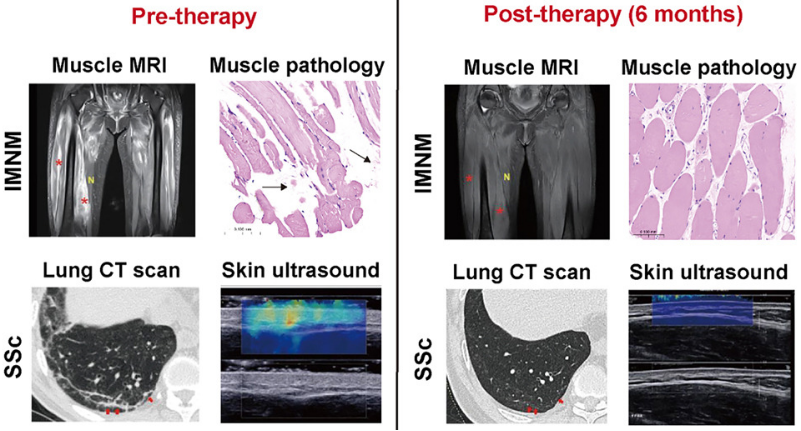
The clinical therapeutic effect of TyU19 on rheumatic immune diseases
The allogeneic universal CAR-T cells—TyU19 developed by the team not only provides a new treatment option for patients with rheumatic immune diseases who currently lack effective treatments, but also demonstrates the significant potential of universal CAR-T cell therapy in terms of efficacy and safety. This research breakthrough signifies a new chapter in the treatment of rheumatic immune diseases. With the deepening of future research and the expansion of clinical trials, there is reason to expect that this therapy will bring benefits to many more patients.
Source:School of Life Sciences
Editor: Philip Nash, Wicky Xu