# Hot Search #
A team led by Professor Li Dali from the School of Life Sciences at East China Normal University (ECNU) has recently developed a novel, ultra-high activity, and miniaturized gene editing tool based on IscB, achieving efficient editing in mice for the first time. This groundbreaking research was published online in the prestigious international academic journal Molecular Cell on August 22, 2024, and was selected as the cover story.

The cover design of the Molecular Cell issue highlighting the ECNU team's work features a captivating Chinese-themed illustration. The main scene depicts two rows of lanterns neatly lined up on a long street, symbolizing DNA. A small child figure (eIscB-D) is seen climbing stairs (eωRNA), expertly replacing lanterns (representing gene editing targets) at specific locations. This swift and precise lantern swapping action embodies the efficiency, miniaturization, and accuracy of the eIscB-D tool. The staircase is artistically rendered with multiple hairpin structures, representing the ωRNA that aids in efficient and precise targeting.
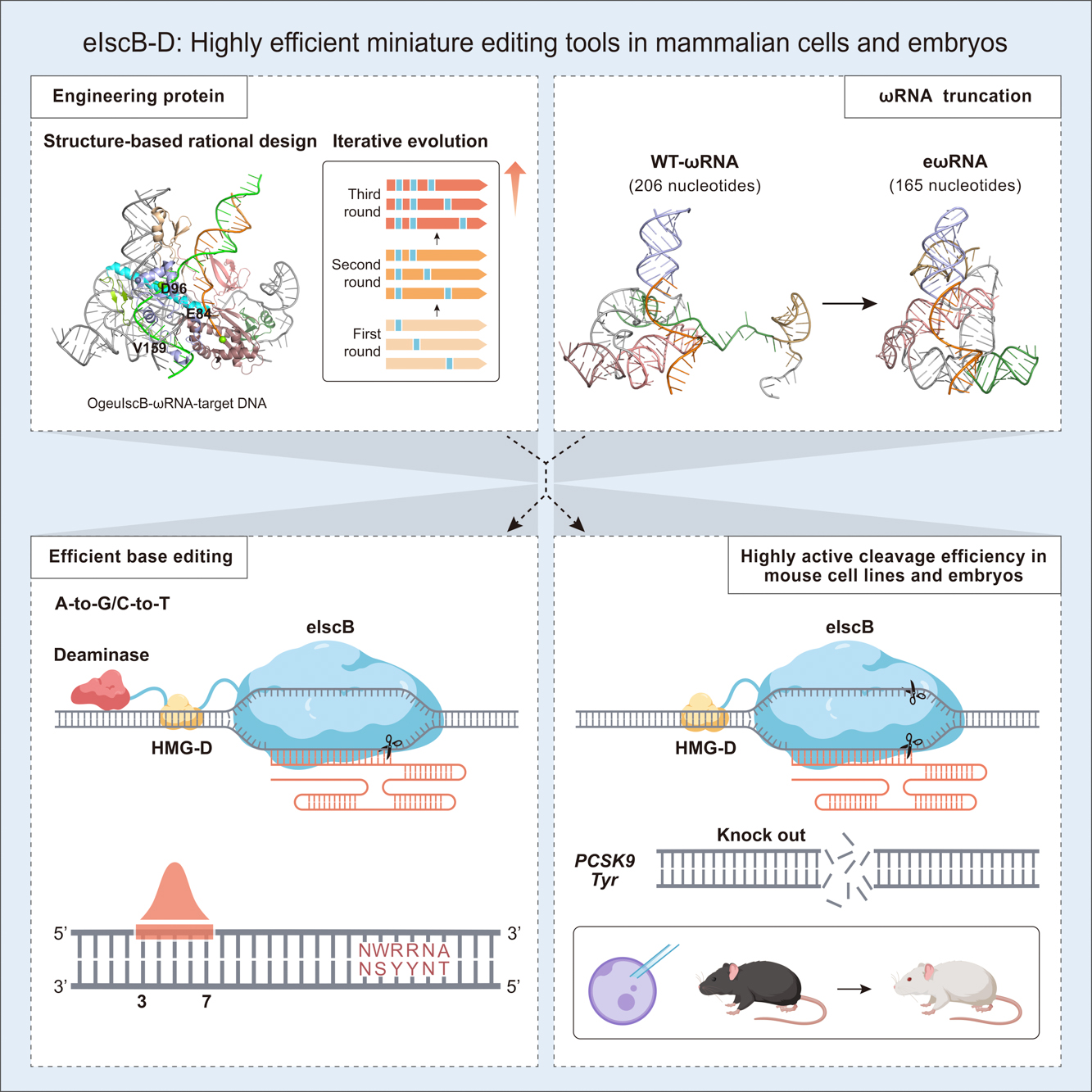
The IscB proteins, as the ancestors of Cas9 endonuclease, hold great promise due to their small size and potential for diverse genome editing. However, their activity in mammalian cells is unsatisfactory. By introducing three residual substitutions in IscB, ECNU researchers observed an average 7.5-fold increase in activity. Through fusing a sequence-non-specific DNA-binding protein domain, the eIscB-D variant achieved higher editing efficiency, with a maximum of 91.3%. Moreover, engineered ωRNA was generated with a 20% reduction in length and slightly increased efficiency. The engineered eIscB-D/eωRNA system showed an average 20.2-fold increase in activity compared with the original IscB. Furthermore, eIscB-D was successfully adapted for highly efficient cytosine and adenine base editing. Notably, eIscB-D is highly active in mouse cell lines and embryos, enabling the efficient generation of disease models through mRNA/ωRNA injection. The study suggests that these miniature genome-editing tools have great potential for diverse applications.

Paper’s Link: Engineering IscB to develop highly efficient miniature editing tools in mammalian cells and embryos - ScienceDirect
Source:School of Life Sciences
Editor: Philip Nash, Wicky Xu